Calculate the Number of Atoms in This Amount of 13c
Express your answer numerically in atoms of carbon. 513 0385 mol.
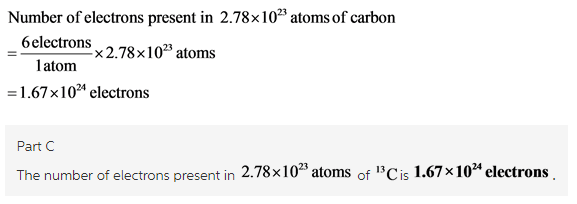
Based On Your Answer In Part B How Many Electrons Are In This Amount Of 13c Home Work Help Learn Cbse Forum
C 167 x 1024 electrons.

. In 13 g of 13C there are precisely Avogadros number of atoms You have specified 300 g thus there are 300 g 1300 g mol. Answer 3241023 13C atom. A 0692 mol 13C.
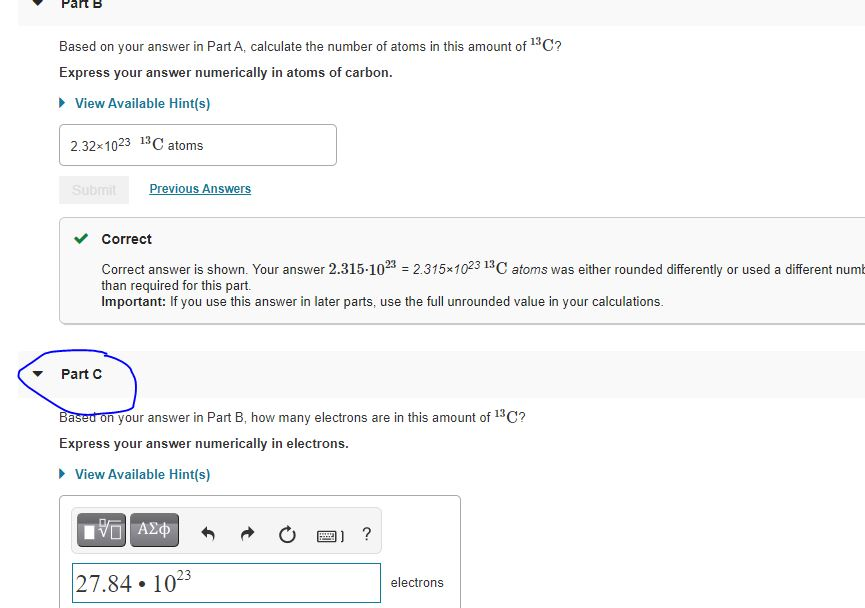
B Based on your answer in Part A calculate the number of atoms in this amount of 13C. View Available Hint s 232x1023 13 C atoms Submit Previous Answers Correct Part Based on your answer in Part B how many electrons are in this amount of C. Answer 0538 mol 13C b Based on your answer in Part A calculate the number of atoms in this amount of 13C.
Part A 13 How many moles of atoms are in 600 g of C. 513 0385 mol. Aug 25 2018.
A How many moles of atoms are in 700 g of 13C. C Based on your answer in Part B how many electrons are in this amount of 13C. Given - number of H atoms in a sample of C3H8 240 1024 atoms To calculate - number of C question_answer Q.
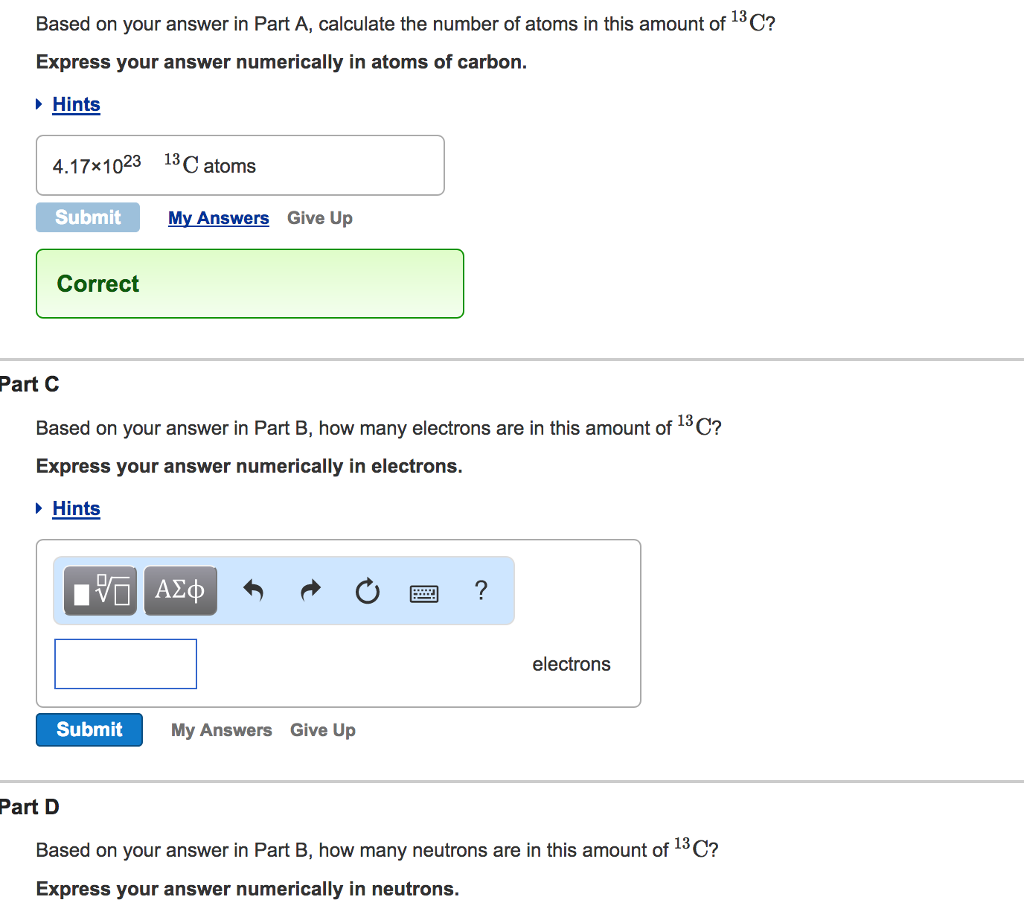
Your answer 2315-1023 2315x1023 13C atoms was either rounded differently or used a different numb than. View Available Hint s 0462 mol 13C Submit Previous Answers v Correct While the value of a mole is the number of atoms of 12C atoms in exactly 120 g of pure 12C the same is not true for other elements or 13 other. Answer 0538 mol 13C.
How many moles of atoms are in 500 of 13C385 moles of atoms are in 500 of 13c. Part D Based on your answer in Part B how many neutrons are in this amount of 13C. Part B Based on your answer in Part A calculate the number of atoms in this amount of 13 C.
B Based on your answer in Part A calculate the number of atoms in this amount of 13C. Answer is 0692 mol 13C Based on your answer in Part A calculate the number of atoms in this amount of 13C. Express your answer numerically in moles.
0154602210²³ 92610²² 13C atoms. Answer 3241023 13C atom c Based on your answer in Part B how. Based on your answer in Part A calculate the number of atoms in this amount of 13C.
7926x10²² 64810²³ neutrons. Based on your answer in Part A calculate the number of atoms in this amount of 13C. A How many moles of atoms are in 700 g of 13C.
There are 602 x 1023 atoms in a mole of anything. Calculate the molar mass of your compound that mass will contain 602 x 1023. B Based on your answer in Part A calculate the number of atoms in this amount of 13C.
How many moles of atoms are in 500 of 13C385 moles of atoms are in 500 of 13c. A How many moles of atoms are in 900 g of 13C. How many moles of atoms are in 900 g of 13C.
A reaction has a standard free-energy change of 1030 kJ mol1 2462 kcal mol1. Express your answer numerically in atoms of carbon. View Available Hint s 232 1023 13C atoms Submit Previous Answers Correct Correct answer is shown.
A 0462 mol 13C. D Based on your answer in Part B how many neutrons are in this amount of 13C. Answer 0538 mol 13C b Based on your answer in Part A calculate the number of atoms in this amount of 13C.
C Based on your answer in Part B how many electrons are in this amount of 13C. Calculate the number of oxygen atoms in 152 g of trinitrotoluene C7H5NO23s. Express your answer numerically in atoms of carbon.
View Available Hint s 324x1023 13C atoms Submit Previous Answers Correct. Based on your answer in Part A calculate the number of atoms in this amount of 13C. A How many moles of atoms are in 700 g of 13C.
B 278 x 1023 13C atoms. D Based on your answer in Part B how many neutrons are in this amount of 13C.
Solved Based On Your Answer In Part A Calculate The Number Chegg Com
Solved Based On Your Answer In Part A Calculate The Number Chegg Com

Solved Based On Your Answer In Part A Calculate The Number Chegg Com

Solved Part B Based On Your Answer In Part A Calculate The Chegg Com
Comments
Post a Comment